Cortico-cortical connections provide the basis for recognition of ‘medial’ and ‘orbital’ networks within the OMPFC. These networks also have distinct connections with structures in other parts of the brain. The orbital network receives sensory inputs from several modalities, including olfaction, taste, visceral afferents, somatic sensation and vision, which appear to be especially related to food or eating. In contrast, the medial network provides the major cortical output to visceromotor structures in the hypothalamus and brainstem. The two networks have distinct connections with areas of the striatum and mediodorsal thalamus. In particular, projections to the nucleus accumbens and the adjacent ventromedial caudate and putamen arise predominantly from the medial network. Both networks also have extensive connections with limbic structures. Based on these and other observations, the OMPFC appears to function as a sensory–visceromotor link, especially for eating. This linkage appears to be critical for the guidance of reward-related behavior and for setting of mood. Imaging and histological observations on human brains indicate that clinical depressive disorders are associated with specific functional and cellular changes in the OMPFC, including activity and volume changes, and specific changes in the number of glial cells.
The cortex on the orbital and medial surface of the frontal lobe is a large and heterogeneous cortical region that covers the medial wall and ventral surface of the frontal lobe. Although the organization and function of this region has been poorly understood, recent anatomical and physiological studies have greatly expanded our knowledge. It can now be recognized that the region as a whole receives highly processed sensory afferents, provides for cortical influence over visceral functions, and participates in high-level cognitive and emotional processes. Several terms have been used to refer to parts of this region, including ‘orbitofrontal’ and ‘anterior cingulate’. In this review, however, the term ‘orbital and medial prefrontal cortex’ (OMPFC) will be used to emphasize the overall function and organization of the region, and its difference from other regions, including most of the cingulate cortex. As discussed in this review, several lines of evidence indicate that the OMPFC can be divided into ‘orbital’ and ‘medial’ prefrontal networks that differ in their intrinsic pattern of cortico-cortical connections and also in their connections with sensory, limbic, striato-thalamic and visceromotor structures in other parts of the brain. These networks appear to represent a separation of sensory and visceromotor functions within the OMPFC.
Starting with classic accounts at the turn of the century, researchers have recognized a number of different cortical fields within the OMPFC in primates and many other species (Brodmann, 1909; Rose and Woolsey, 1948). Although the prefrontal cortex as a whole shows considerable variation across species, especially in the amount of granular versus agranular cortex, similarities in the position and connections of orbital and medial areas indicates that the OMPFC is relatively comparable across species (Uylings and Van Eden, 1990; Petrides and Pandya, 1994). Comparative cytoarchitectonic studies of the prefrontal cortex may allow a further extension of the correlation from extensively studied animal species to humans, where experimental data are scarce. This is of particular relevance to human neuroimaging studies where knowledge of the anatomy and physiology of brain areas is crucial for sound interpretation of the data.
Human
Brodmann's human map resembles his monkey and other maps (Fig. 3A), although he remarked that his monkey and human maps are not identical (Brodmann, 1909; Garey, 1994). He particularly noted that the homologies between human and monkey areas are unclear in the frontal (prefrontal) and cingulate regions. In the human map the medial prefrontal cortex is occupied mainly by areas 24, 25, 32 and 10, although Brodmann particularly stated that area 32 is not homologous to area 32 in the monkey map. In one map that has been republished in several subsequent reviews (Petrides and Pandya, 1994), he included an area 12 on the medial wall anterior to the corpus callosum, but this is not found in his monograph (Brodmann, 1909) or preceding journal articles (Brodmann, 1905, 1908). Where illustrated, this area 12 is clearly different from area 12 in Walker's monkey map (Walker, 1940). The lateral orbital area that is usually denoted as area 12 in monkeys appears to correspond to area 47 in Brodmann's human map (Petrides and Pandya, 1994). Brodmann did not illustrate the rest of the orbital surface in detail, but most of it appears to have been denoted as area 11.
Several investigators have proposed modifications to Brodmann's original parcellation of the human OMPFC. A report by Beck recognized the cortex of the human gyrus rectus as area 14 (Beck, 1949). The same report also noted the agranular to granular gradient on the orbital surface and designated the posterior, dysgranular orbital cortex as area 13. Both of these modifications brought Brodmann's map into closer accord with that of Walker (Walker, 1940). Semendeferi and co-workers also recognized area 13 on the posterior orbital surface of several different primate species, including those of macaque monkeys and humans (Semendeferi et al., 1998). Petrides and Pandya have made an attempt at formally reconciling the Brodmann and Walker labeling schemes in human and monkey prefrontal cortex (Fig. 3B) (Petrides and Pandya, 1994). They use the term area 47/12 to refer to the lateral orbital areas in both humans and monkeys, preserve area 32 anterior to the genu of the corpus callosum, area 24 on the anterior cingulate cortex and area 25 on the posterior medial wall. More recent studies have elaborated on similar themes even though they do not always use similar nomenclature. Using immunohistochemical as well as cytoarchitectonic evidence, Hof and co-workers divided the orbital cortex into eight distinct areas, although they denoted the areas by letters that indicate their position (Fig. 3C) (Hof et al., 1995). Because of this, it is difficult to compare their map with those of other workers.
There is now general agreement (Petrides and Pandya, 1994; Semendeferi, 1998) that the cytoarchitecture of the orbital cortex is similar in humans and non-human primates and the differences in Brodmann's and Walker's maps have been largely reconciled. The broad parallels in the organization of the OMPFC in monkeys and humans suggest that cytoarchitectonic areas that are identified anatomically and physiologically in the macaque brain may have counterparts in the human as well. Lacking anatomical connectivity data, the only avenue to define such areas in humans is to study staining patterns in post-mortem material just as was done in monkeys.
Recently we have analyzed sections of the human OMPFC prepared with five different stains in order to apply the architectonic analysis that was done in monkeys by Carmichael and Price (Carmichael and Price, 1994) (Fig. 4; D. Öngür and J.L. Price, unpublished observations). Almost all of the areas that were recognized in monkeys were also identified in the human, although there are some changes in the relative size of many of the areas. Because area 32 in Brodmann's human map does not correspond to area 32 in the monkey, two areas were distinguished in the medial prefrontal cortex. Area 32h corresponds to the area in the human map, dorsal and rostral to the corpus callosum. In contrast, area 32m, corresponding to the area in monkeys, is recognized in the caudoventral part of the medial wall, adjacent to area 25. The granular area 10m appears to have expanded so greatly, occupying most of the medial wall, that it has been divided into area 10r rostrally and 10m caudally.
The orbital cortex has been recognized to have the same areas as in the monkey (Fig. 4). Areas 14r and 14c are located at the ventromedial edge of the gyrus rectus, while areas 13a and 13b occupy the caudal part of the olfactory sulcus. Areas 13m and 13l occupy the central part of the orbital cortex caudally, and areas 11m and 11l have the same position more rostrally. Area 10o is found on the orbital surface of the frontal pole. Subdivisions of area 47/12 (47/12m, 47/12l and 47/12r) occupy the lateral orbital cortex. The apparent homolog of monkey area 12o (12 orbital) is situated within the rostral continuation of the superior limiting sulcus into the frontal lobe, and has been renamed area 47/12s (47/12 sulcal). As in monkeys, the agranular insula extends onto the caudal orbital surface and can be divided into areas Iam, Iapm, Ial and Iai. Area Iai, in particular, extends into the rostral extension of the superior limiting sulcus, dorsomedially adjacent to area 12s.
Anatomical Connections of the OMPFC
The OMPFC has extensive connections with other parts of the brain, including the mediodorsal nucleus of the thalamus, several sensory areas or systems, virtually all limbic structures and areas, the ventromedial striatum and the hypothalamus and brainstem. In addition, there are intrinsic cortico-cortical connections, which are sufficiently structured that they allow the definition of distinct ‘networks' within the OMPFC. Because these networks provide an organizational principle that can be used to analyze the extrinsic connections, they will be summarized first, and then the sensory, limbic, striatal and visceromotor connections will be considered.
Intrinsic Corticocortical Connections
The intrinsic corticocortical connections of the OMPFC have been analyzed in a number of experiments with small injections of retrograde and anterograde axonal tracers that were restricted to single architectonic areas (Carmichael and Price, 1996). This analysis indicated that the areas of the OMPFC can be divided into two groups or networks, one restricted to the orbital cortex and the other involving the medial cortex and some orbital areas (Fig. 5). Tracer injections in most orbital areas labeled many cells or axons in other orbital areas but only a few in the medial wall. Within the orbital cortex, a few areas (Iai, 12o, 13a and 11m) were more weakly labeled than other orbital areas, and in some cases were conspicuously lacking in label. In contrast, injections in the medial wall areas labeled cells or axons not only in medial areas 24, 25, 32 and 10m, and in the ventromedial areas 14r and 14c, but also in the orbital areas Iai, 12o, 13a and 11m. Furthermore, injections in these select orbital areas produced label in the medial wall areas.
Based on these results, most of the areas on the posterior, central and lateral orbital surface (agranular insular areas Ial, Iam, Iapl and Iapm, and orbital areas 13b, 13l, 13m, 11l, 12r, 12m and 12l) were defined as the ‘orbital prefrontal network’ (Fig. 5). As discussed below, this network receives inputs related to several sensory modalities and appears to be involved in sensory integration. A separate group that included all the areas on the medial wall (areas 25, 32, 14r, 14c, 24a and 24b) and the related areas on the orbital surface (areas 11m, 13a, Iai and 12o) were together labeled the ‘medial prefrontal network’. Data summarized below have indicated that this network provides the origin of most of the descending projections to the hypothalamus and brainstem.
Although the two OMPFC networks are clearly distinct, a number of pathways provide communication between them. For example medial network areas 14r, 14c and 12o also have connections with areas of the orbital network. Area 11m, which projects mainly to medial network areas, receives a substantial input from adjacent area 11l, which in turn is innervated by posterior orbital areas that are part of the orbital network (Carmichael and Price, 1996). Thus the sensory-receptive orbital network is connected to the visceromotor medial network, providing a basis for sensory–motor linkage.
Sensory Inputs to the OMPFC
In the words of Nauta, ‘all major sensoria find some form of representation in the prefrontal cortex, however abstract or compounded’ (Nauta, 1971). Indeed, visual, auditory, somatosensory, olfactory, and gustatory inputs to the OMPFC have been reported in macaque monkeys (Pandya and Kuypers, 1969; Jones and Powell, 1970; Chavis and Pandya, 1976; Mesulam and Mufson, 1982; Kawamura and Naito, 1984; Petrides and Pandya, 1988; Barbas, 1988; Seltzer and Pandya, 1989; Rolls et al., 1989; Carmichael et al., 1994; Hackett et al., 1999; Romanski et al., 1999). Similar inputs from olfactory, gustatory, somatosensory and visual sensory areas have also been reported in rats (Guldin and Markowitsch, 1983; Miller and Vogt, 1984; Turner and Zimmer, 1984; Price et al., 1991a; Conde et al., 1995; Datiche and Cattarelli, 1996; Reep et al., 1996).
Experiments with injections in single OMPFC areas in macaques show that projections from different sensory modalities terminate in restricted areas of the orbital network, especially in the caudal and lateral orbital cortex: visual inputs in area 12l, somatosensory inputs in area 12m, visceral inputs in areas Ial and Iapm, olfactory inputs in areas Iam and Iapm, and gustatory inputs in the primary gustatory cortex (G) (Fig. 5) (Carmichael and Price, 1995a). All of these areas are in the caudal and lateral part of the orbital network, and projections within this network converge on more rostral areas, providing for integration between several sensory modalities, and with limbic influences (see below) (Carmichael and Price, 1995a). The central orbital cortex is the first part of the central nervous system where olfactory and gustatory inputs converge, and thus it is probably involved in generating the sensation of flavor associated with different foods. Indeed, many of the other sensory inputs, such as the visceral afferents that parallel the gustatory pathway through the ‘parvicellular’ part of the ventral posterior medial thalamic nucleus, also appear to be related to food or feeding. Even the somatic sensory inputs appear to arise from the hand and face regions of the somatic sensory cortical areas (Carmichael and Price, 1995a). In contrast to these sensory inputs to the orbital network, there are very few direct sensory inputs to the medial network.
Studies in both rats and monkeys show not only that cells in the OMPFC respond to multisensory stimulation (Rolls, 1997; Rolls et al., 1999), but also that the responses are often related to the affective value of the stimulus as well as its sensory properties. For example, the ensemble firing of neurons in the rat orbital cortex during an associative learning task codes better for the reward properties of a smell than for the identity of the smells (Schoenbaum and Eichenbaum, 1995). Neurons with firing properties such as these are found in the macaque orbital cortex in response to visual and flavor stimulation as well (Rolls, 1984). Rolls and colleagues have shown that neurons that fire strongly at the sight of a desirable food item while the animal is hungry have much less response when the animal is satiated for the same food item (Critchley et al., 1996). Many of these neurons are still responsive to other appetizing food items. The work of Schultz and colleagues indicates that the OMPFC is part of a wider circuit including limbic structures as well as the ventral striatum and midbrain dopamine cells that respond to reward associations of sensory stimuli. However, the OMPFC is unique among these areas in its coding of the identity of the sensory stimuli as well as their rewarding properties (Schultz et al., 1998).
Connections with Limbic Structures
In addition to receiving processed sensory inputs, the OMPFC is also intimately connected with limbic forebrain areas (Fig. 6). In rats and cats, as well as monkeys, the OMPFC has largely reciprocal connections with the basal, accessory basal, and lateral nuclei of the amygdala, the subiculum, and the entorhinal and perirhinal cortex (Krettek and Price, 1977; Swanson, 1981; Ray and Price, 1992; Barbas and Blatt, 1995; Carmichael and Price, 1995b). In monkeys, these connections as a whole are not selective for either the medial or the orbital network. The projection from the basal amygdaloid nucleus, however, is divided between the two networks in an organized manner (Carmichael and Price, 1995b). The ‘medial’ network (including the orbital areas Iai and 12o) is primarily connected to the ventrolateral part of the basal nucleus, while areas of the orbital network are connected to the ventromedial part of the nucleus. The entorhinal, perirhinal and temporal polar cortices have extensive projections, especially within the orbital cortex, although no organization has been discerned within these systems relative to the two networks. The projection from the subiculum arises primarily from the rostral subiculum, especially from the region at the border between the subiculum and CA1. The fibers terminate in areas associated with the medial prefrontal network, specifically in the caudal part of the medial wall and the medial part of the orbital cortex (Barbas and Blatt, 1995; Carmichael and Price, 1995b).
Connections with the Striatum and Thalamus
Studies of the projections from the OMPFC to the striatum have shown that distinct regions of the prefrontal cortex project to distinct portions of the ventromedial striatum (Fig. 7). In rats, the OMPFC as a whole projects to the ventromedial part of the striatum, including the nucleus accumbens, the medial part of the caudate nucleus and the ventral part of the putamen (Philipson and Griffiths, 1985; Berendse et al., 1992; Brog et al., 1993). Within the OMPFC, however, different areas project to different striatal regions. For example, the infralimbic area projects strongly to the shell of the nucleus accumbens, while the prelimbic area projects to the core of the nucleus accumbens and the medial part of the caudate nucleus. Lateral areas, such as the agranular insular cortex, project to a contiguous ventrolateral part of the striatum, including the ventral putamen. Notably, the lateral orbital area, in the middle of the orbital cortex, has been reported to project to the central part of the caudate/putamen, out of the sequence of projections from adjacent areas (Berendse et al., 1992).
In monkeys the striatal projections from the OMPFC appear to be organized according to the medial and orbital prefrontal networks (Fig. 7). As in rats, area 25 (infralimbic) appears to be the only area that projects substantially to the shell of the nucleus accumbens, but it and other areas in the medial network project to a ventromedial zone in the rostral striatum encompassing the medial caudate nucleus, the core of the nucleus accumbens and the ventral putamen (Haber et al., 1995; An et al., 1997) (also A. Ferry, D. Öngür, X. An and J.L. Price, unpublished observations). Orbital areas that are related to the medial network (13a, 12o and Iai) appear to have similar projections. In contrast, the other orbital areas within the orbital network mainly project to a central band in the rostral striatum including the ventrolateral caudate, the dorsal edge of the nucleus accumbens and the medial putamen (An et al., 1997) (also A. Ferry et al., unpublished observations). It is notable that the ventromedial part of the striatum, which receives input from the medial network, also receives fibers from the amygdala and other limbic structures, while the central striatal region, which is related to the orbital network does not (Russchen and Price, 1984; Russchen et al., 1985).
The ventromedial striatal regions that receive fibers from the OMPFC in turn project to the ventral pallidum, the ventral and rostral extension of the globus pallidus. The ventral pallidum then projects to the medial part of the mediodorsal thalamic nucleus (MDm), which is reciprocally interconnected with the OMPFC (Fig. 7). Remarkably, the distinction between the orbital and medial networks is also seen in the connections with MDm, such that areas within the two networks are reciprocally related to different subdivisions of this nucleus. Thus, areas 32, 24, 14, 13a and Iai, all belonging to the medial network, are primarily connected to the paramedian edge and caudodorsal part of the medial part of MDm (Ray and Price, 1993). In contrast, areas of the orbital network are all primarily connected to the fibrous part of MDm that is more centrally located within MD as a whole.
Although all of the projections have not yet been analyzed in sufficient detail, it appears possible, therefore, that there are distinct striato-pallido-thalamo-cortical circuits related to the medial and orbital networks. The ventral striato-pallidal circuit is comparable to the parallel dorsal striato-pallido-thalamo-cortical circuit that involves the dorsolateral striatum, globus pallidus, ventroanterior and ventrolateral thalamic nuclei and the DLPFC. However, where the dorsal circuit has been implicated in motor functions, it is likely that the ventral circuit is involved in reward-guided choice behavior (Schultz et al., 1998).
It should be noted that the intralaminar nuclei of the thalamus, especially the medial and midline nuclei, also have extensive connections with both the ventromedial striatum and the OMPFC (Berendse and Groenewegen, 1990, 1991). As yet, however, these have not been analyzed in relation to the medial or orbital prefrontal networks.
Connections with the Hypothalamus and Brainstem
It has been recognized since the last decades of the 19th century that the cerebral cortex exerts an influence over visceral function and the autonomic nervous system in particular. A large number of studies have described cortical control over cardiovascular, gastric and respiratory systems in monkeys, dogs, cats, rabbits and rats (Hoff and Green, 1936; Smith, 1945; Fulton, 1949; Penfield and Rasmussen, 1950; Kaada, 1951; Wall and Davis, 1951). Specifically, a region of cerebral cortex including the anterior cingulate gyrus, the medial wall ventral to the corpus callosum, the posterior orbital surface, the agranular insula and the temporal polar cortex was shown affect a wide variety of physiological systems under autonomic control (Kaada, 1960). As discussed below, most of this output arises in areas of the medial prefrontal network, which can be considered a ‘visceromotor’ system.
In his review of the literature on cortical control of the autonomic nervous system, Neafsey noted that there is not a simple relationship between cortical activity and the indices being monitored (Neafsey, 1990). Depending on the location of the electrode and the current intensity, cortical stimulation produces variable changes in heart rate or blood pressure, and some recent studies even report intermingled pressor and depressor sites (Kaada et al., 1949; Lofving, 1961; Terreberry and Neafsey, 1987; Fisk and Wyss, 1997). It is probable that these physiological studies reveal distinct output pathways from closely positioned areas of cingulate, subcallosal and posterior orbital cortex.
The major autonomic related structures that receive fibers from the OMPFC are the hypothalamus and periaqueductal gray (PAG) (Hardy and Holmes, 1988; Cechetto and Chen, 1990), although in rats there also appear to be cortical projections to more caudal brainstem structures (Terreberry and Neafsey, 1987; Neafsey 1990; Hurley et al., 1991). Within these structures, different nuclei or columns (in the PAG) have been associated with distinct functions as diverse as aggression, autonomic control, feeding, drinking, sexual behavior, thermoregulation and anti-nociception (Swanson, 1987). Such functions may occur separately, but they are often evoked together as parts of coordinated coping strategies. For example, stimulation of the lateral column of the PAG by excitatory amino acids generates tachycardia, hypertension and non-opioid analgesia as part of an overall confrontational behavioral stance. In contrast, stimulation of the ventrolateral column of the PAG generates bradycardia, hypotension and opioid analgesia within a quiescent behavioral stance (Bandler and Shipley, 1994). By its influence on such responses the OMPFC goes well beyond reflexive effects to evoke a coordinated array of responses that define coping strategies in response to a challenge.
It has been known for some time that in rats there are anatomical projections to the hypothalamus and brainstem from both medial and lateral (orbital) prefrontal areas (Terreberry and Neafsey, 1987; Neafsey 1990; Hurley et al., 1991; Price et al., 1991b; Bandler and Shipley, 1994). In monkeys, it has recently been shown that the projections to the hypothalamus and PAG arise almost entirely from areas in the medial prefrontal network (Fig. 5) (An et al. 1998; Öngür et al., 1998a). Cells labeled by retrograde tracer injections into the PAG are almost entirely restricted to the medial network and closely related areas (e.g. area 9 in the dorsomedial prefrontal cortex). Cells that project to the hypothalamus are also primarily located in the medial network, although there is also a light projection to the posterior hypothalamus from agranular insular areas that are otherwise considered part of the orbital network. Recent observations in rats indicate that the cortical projections to the PAG arise in the medial wall and in the far lateral dorsolateral orbital area and dorsal agranular insular area, but not from intervening orbital areas in the dorsal bank of the rhinal sulcus (N. Floyd, J.L. Price, K. Keay and R. Bandler, unpublished observations). These areas appear to be homologous to the medial network defined in monkeys. Outside the OMPFC, the only cortical areas in monkeys that project to the hypothalamus and PAG are parts of the temporal pole and dysgranular insula. Similar cortical regions also provide the cortical efferents to the dopaminergic, serotonergic and noradrenergic brainstem nuclei in rats and monkeys (Nauta, 1971; Sesack et al., 1989).
The medial prefrontal network can be subdivided into several regions that have distinctive projections to different portions of the hypothalamus and PAG (Fig. 5) (An et al. 1998; Öngür et al., 1998a). Anterograde tracer injections in medial wall areas 25 and 32 result in strong label in most hypothalamic regions, including in particular the medial hypothalamus. The same areas, along with medial area 10m, project strongly to the dorsolateral column of the PAG. Dorsomedial areas 24b and 9 project strongly to the dorsal hypothalamus and to the lateral PAG column, while orbital areas 13a, 12o and Iai project to the lateral hypothalamus and the ventrolateral column of the PAG. Similar subdivisions can be recognized in rats (N. Floyd et al., unpublished observations). There are also substantial ascending and descending connections between the hypothalamus and PAG, and it is probable that the portions of the hypothalamus and PAG that receive similar cortical inputs are interconnected (Beitz, 1981; Venning et al., 1991). Hypothalamic and PAG projections to the parabrachial nucleus and other brainstem regions may also be organized along the same lines (Krout et al., 1998).
Functions of the OMPFC
Sensory—Motor Linkage in the OMPFC
In his synthesis of the physiological studies with anatomical data from the rat, Neafsey proposed that prefrontal cortex can be divided into visceral sensory areas on the ventrolateral (orbital) surface and visceral motor areas on the medial wall (Neafsey, 1990). These are situated on either side of the dorsal somatic sensory and motor cortical areas. This concept is useful in interpreting the anatomical data on the macaque OMPFC as well since the sensory inputs into the OMPFC mainly reach the orbital network while the subcortical outputs to visceromotor structures mainly arise from the medial network. (The sensory– motor distinction appears not to lie along a simple mediolateral axis, however, since the medial network areas Iai and 12o lie on the lateral orbital surface.) It is important to note that while the two networks are distinct, there are a number of points of interaction by which sensory information may be transferred from the orbital to the medial network. Many of these pathways are through areas at the ventromedial corner of the frontal lobe, especially areas 13a, 14r, 14c and 11m.
Fuster proposed a similar sensory-motor transfer system through the DLPFC (Fuster, 1997). In this scheme, highly processed information from sensory association areas in the parietal and temporal cortex converges onto the DLPFC. The DLPFC then produces behaviors based on this sensory input via projections to premotor and motor areas. The difference with the OMPFC is that the output is primarily to visceromotor control. Interestingly, this system also appears to have a major role in the guidance of behavior.
Guidance of Behavior by the OMPFC
Behavioral studies suggest that in addition to linking sensory and visceromotor activity, the OMPFC also participates in the guidance of emotional behavior. As discussed below, these two functions appear to be closely linked. Monkeys with lesions of the OMPFC show abnormal food preferences (Ursin et al., 1969; Butter et al., 1969; Baylis and Gaffan, 1991) and also have altered aggressive and aversive responses to threatening stimuli (Butter and Snyder, 1972). Many of the behavioral effects of OMPFC lesions are remarkably similar to those seen following amygdala lesions, indicating that both of these structures are involved in circuits controlling emotional and social behaviors (Kling, 1972). For example, in free-ranging settings animals with prefrontal cortex lesions, like those with amygdala lesions, lose their position in the social hierarchy (Butter and Snyder, 1972), or fall out of the troop altogether and die in solitude (Myers et al., 1973).
These behavioral studies have remained somewhat segregated in the literature from studies on the visceral functions of the OMPFC, but this may be deceiving. In a review full of remarkable insights, Nauta posited that the prefrontal cortex is informed about most sensory stimuli impinging on the sensory organs of the body, and that the OMPFC uses this information to obtain the most desirable and rewarding outcomes for the organism (Nauta, 1971). Nauta emphasized that the visceral sensory inputs and visceromotor outputs are particularly important in this regard since they provide information about the status of the internal milieu and generate bodily reactions to alter that status. These bodily reactions are then relayed to the prefrontal cortex as new information on the state of the body, forming a loop where the OMPFC is constantly monitoring and altering that state. Based on these ideas, Nauta suggested that animals with lesions of the prefrontal cortex experience an ‘interoceptive agnosia', an inability to determine how the body is reacting when faced with contrasting behavioral options. Since the organism is deprived of the ‘navigational markers' it would normally use to decide which action is the most desirable, this agnosia then leads to a variety of emotional, social and cognitive deficits.
These ideas that Nauta distilled from anatomical studies have received important support from clinical studies in humans. Observations about lesions of the OMPFC have a long history, starting with Harlow's report on his patient Phineas Gage, a respected railroad construction foreman who after a work accident sustained damage to most of the medial wall and orbital surface of the frontal lobe (Harlow, 1868). After the accident, Gage underwent personality changes and started showing impulsive, socially inappropriate behavior. Despite apparently possessing all of his intellectual faculties, he lost his job, strained his family ties and was left a changed person. In another early study, Welt (Welt, 1888) described a patient with a penetrating frontal wound who developed personality changes very similar to those suffered by Gage, although they later resolved (Benton, 1991). In her study, Welt also reviewed and synthesized many other reports of behavioral change following frontal lobe injury and concluded that the most consistent locations of damage associated with disinhibited, dysfunctional behavior were the orbitofrontal gyri. In this century, frontal leukotomies performed on psychiatric patients to decrease anxiety, depression and a host of other problems generated renewed interest in frontal lobe lesions, although they were of uncertain clinical value. The clinical picture following this procedure was a loss of emotional regulation that manifested itself in various ways. Some patients showed apathy toward most things that would elicit emotional reactions from normals, while others became disinhibited and inappropriately outgoing (Valenstein, 1986).
More recently, Damasio and colleagues showed in a series of studies that patients with specific OMPFC damage give deficient visceral responses to disturbing or exciting images, as demonstrated with galvanic skin responses (GSRs). Particularly in their prototypical patient E.V.R., this lack of visceral/emotional response is coupled with an inability to make appropriate life decisions (Damasio et al., 1990). Bechara and colleagues demonstrated this inability in a card game intended to model daily-life decision making (Bechara et al., 1996, 1997). In this game, which uses play money, normal controls quickly learn to navigate their way without losing money, even before they develop conscious awareness of this fact and before they employ explicit strategies for winning. This quick adaptation phase is characterized by anticipatory GSRs prior to each unfavorable move. E.V.R. and other OMPFC-lesioned patients display a different pattern: they are deficient in their anticipatory GSRs and keep making bad moves that look to them to be lucrative in the short term. Much like in real life, the patients eventually go bankrupt.
In an effort to understand this complex behavioral deviation, Damasio has proposed a ‘somatic marker hypothesis' (Damasio, 1994). This hypothesis echoes earlier theories by William James, who equated emotional experience with the visceral reactions of the body (James, 1890); by Schachter and Singer, who suggested that the dissonance between cognitive states and visceral reactions may trigger conscious perceptions of emotion (Schachter and Singer, 1962); and most specifically by Nauta, who suggested that navigational markers produced by the prefrontal cortex guide behavior (Nauta, 1971). Damasio suggests that CNS-generated changes in the internal milieu of the organism serve to color our daily experiences and tag certain situations or outcomes as positive or negative. The OMPFC may serve to evaluate external events and to generate autonomic or somatic changes in the body, thus signaling to the organism that a given behavioral option is risky or undesirable. Damasio suggests that the inability of E.V.R. and other patients with damage to the OMPFC to generate these kinds of internal reactions renders them dysfunctional in making many small and large decisions on a daily basis.
Mood Disorders and the OMPFC
Several lines of evidence indicate a role for the prefrontal cortex in emotional behavior. Disinhibited or apathetic behavior has been reported in monkeys and humans following damage to the ventral prefrontal cortex. The OMPFC is related to fearand reward-related circuits through its connections with the amygdala and other medial temporal lobe structures and the ventral striatum. The OMPFC also projects to sites in the diencephalon and brainstem that mediate the endocrine, visceral and somatic changes accompanying emotional behavior. However, the relationship between emotional behavior and the setting of mood is not always clear. Mood represents the longer-term internal experience of an organism and it colors one's sense of self and relation to the external world. It is influenced by but distinct from fleeting emotional or motivational experiences. It is difficult to study mood states in experimental animals but the OMPFC has been implicated in mood disorders in humans.
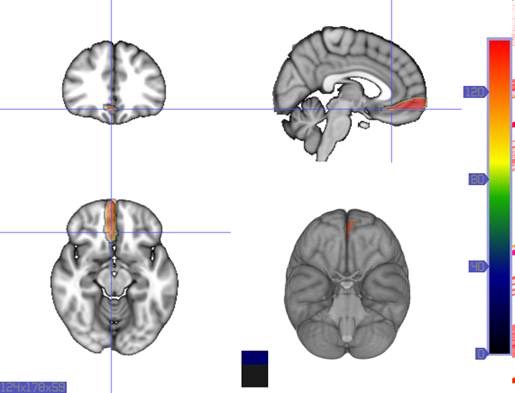
Major depressive disorder (MDD) and bipolar disorder (BD), together known as mood disorders, are the most common pathological alterations of mood and emotion in humans. The use of in vivo neuroimaging techniques has helped identify a circuit of forebrain areas that is altered in activity and structure in mood disorders. Within this circuit, the OMPFC, mediodorsal thalamus and amygdala were found to have increased blood flow in patients with familial pure depressive disorder (Drevets et al., 1992). This classification denotes a subset of MDD patients with positive family history for MDD or BD, no family history for alcoholism and no history of psychotic or manic episodes.
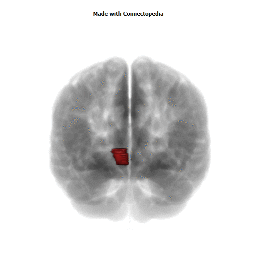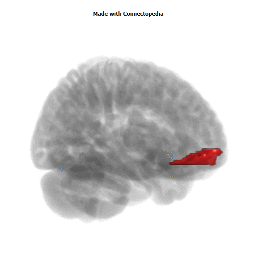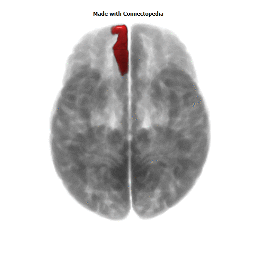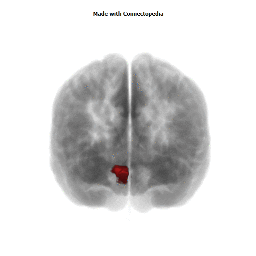
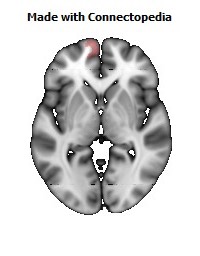
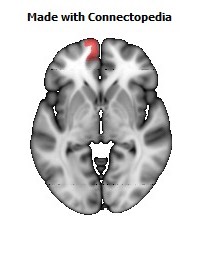
Morphological changes in the depressed brain have been difficult to identify but they may provide indications of the neural deficit underlying mood disorders. One such change has been found in a small area in the subgenual prefrontal cortex. Unlike other parts of the OMPFC, this area shows a relative decrease in blood flow in patients with MDD and BD (Drevets et al., 1997). It corresponds to the first gyrus ventral to the genu of the corpus callosum [the subgenual part of Brodmann's area 24 (area sg24)]. On magnetic resonance imaging (MRI)-based volumetric measurements, the subgenual prefrontal cortex is actually 40% smaller in depressed patients and this at least partially accounts for the observed decrease in blood flow.
Although the basis for the tissue shrinkage in area sg24 is not clear, examination of autopsy brains from subjects with MDD or BD has indicated that there is a specific cellular difference in this area and possibly in other parts of the OMPFC. Cell counts in these areas have shown that the number of neurons is approximately normal, but that there is a specific reduction in the number and density of glial cells (Fig. 8) (Öngür et al., 1998b; Rajkowska et al., 1999). The reduction is especially prominent in subjects with a family history of MDD or BD. A reduction in glia was not found in the primary somatosensory cortex in the post-central gyrus in the same cases. Recently, however, a similar reduction has been found in the amygdala of depressed subjects (M. Bowley, D. Öngür and J. Price, unpublished observations).
The involvement of the OMPFC in mood disorders fits well with its proposed role in eating and visceromotor function. The most recent version of the Diagnostic and Statistical Manual, DSM-IV, includes disturbances in eating and sleeping patterns as well as mood changes among its diagnostic criteria for mood disorders, and eating disorders have a very high rate of co-morbidity with MDD. Mood disorders are often associated with psychomotor retardation of movement and neuroendocrine changes as well. Finally, MDD is associated with increased mortality from cardiovascular disorders and sudden cardiac death (Glassman and Shapiro, 1998). It is clear that mood disorders are not simple pathological alterations of emotional processing but that they bring along a spectrum of endocrine, visceral and somatic changes. This should not be surprising given the close association between emotional processing and CNS control over the internal state of the body.
The Organization of Networks within the Orbital and Medial Prefrontal Cortex of Rats, Monkeys and Humans
D. Öngür and J.L. Price, Cerebral Cortex 2000 10(3) :206-219